RESEARCH
Main theme: Optimization of Oral Dosage Forms
Oral formulations are the most versatile and convenient drug dosage form. Recently, the proportion of oral formulations in clinical use has been decreasing due to the development of macromolecular drugs, including peptides and antibodies, but the development of oral formulations as pharmaceuticals is extremely important from the perspective of patient QOL (Quality of Life). Therefore, we are solving various problems in oral formulations and establishing new formulation strategies. We also actively conduct collaborative research with other research institutions.
1. Evaluation of drug absorption from oral dosage forms
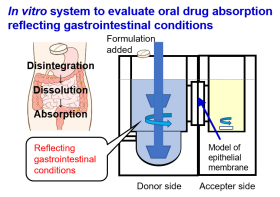
Drug absorption from the gastrointestinal tract is determined by the dissolved concentration of the drug at the absorption site, its permeability to the small intestinal epithelial cell membrane, and residence time. Therefore, evaluating the water solubility and membrane permeability of drugs is important. However, because drug dissolution from administered formulations can vary greatly due to differences in the physiological conditions in the gastrointestinal tract (such as the difference in secretion of gastric juice and the food state), individual evaluation of solubility and membrane permeability alone is insufficient. Therefore, we have established several in vitro systems that reflect the physiological conditions in the gastrointestinal tract and have demonstrated their usefulness and applicability. Currently, we are conducting research that can contribute to developing oral formulations and solving clinical problems to improve the system further.
Publications
Kataoka M, et al. Pharm Res. 2003. 20(10):1674-1680. doi: 10.1023/a:1026107906191.
Kataoka M, et al. Eur J Pharm Biopharm. 2016. 101:103-111. doi: 10.1016/j.ejpb.2016.02.002.
Masada T, et al. J Drug Deliv Sci Technol. 2020. 102747. doi: 10.1016/j.jddst.2021.102747.
Masada T, et al. Pharmaceutics. 2021. 13(8):1136. doi: 10.3390/pharmaceutics13081136.
and others
2. Improving oral absorption of poorly water-soluble drugs
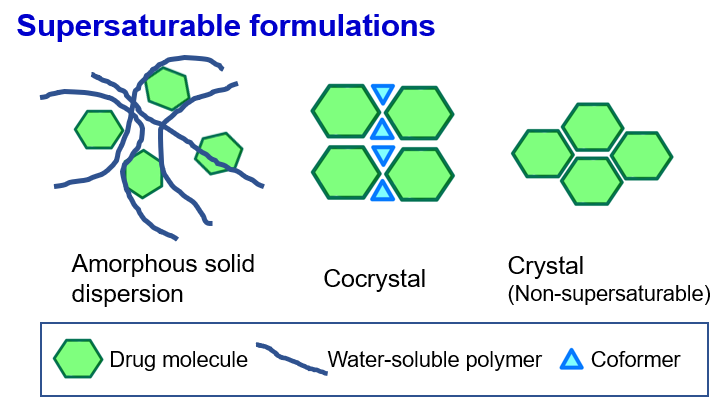 Recently, various supersaturated dissolution techniques have been applied
to improve the oral absorption of poorly water-soluble drugs. Supersaturated
dissolution is a phenomenon in which the dissolved concentration exceeds
the thermodynamic solubility (equilibrium solubility with stable crystals).
Oral drug absorption is expected to significantly improve when the supersaturated
dissolution of poorly water-soluble drugs occurs in the gastrointestinal
tract. We are focusing on supersaturable techniques such as amorphous solid
dispersions and cocrystals and conducted research not only on the usefulness
of these technologies but also on the control and optimization of drug
absorption behavior. We are also researching improving the oral absorption
of poorly water-soluble drugs by using liposomes (spherical vesicles with
a lipid bilayer).
Recently, various supersaturated dissolution techniques have been applied
to improve the oral absorption of poorly water-soluble drugs. Supersaturated
dissolution is a phenomenon in which the dissolved concentration exceeds
the thermodynamic solubility (equilibrium solubility with stable crystals).
Oral drug absorption is expected to significantly improve when the supersaturated
dissolution of poorly water-soluble drugs occurs in the gastrointestinal
tract. We are focusing on supersaturable techniques such as amorphous solid
dispersions and cocrystals and conducted research not only on the usefulness
of these technologies but also on the control and optimization of drug
absorption behavior. We are also researching improving the oral absorption
of poorly water-soluble drugs by using liposomes (spherical vesicles with
a lipid bilayer).
Publications
Mizoguchi M, et al. J Pharm Sci. 2018. 107(9):2404-2410. doi: 10.1016/j.xphs.2018.05.009.
Kataoka M, et al. J Pharm Sci. 2019. 108(8):2580-2587. doi: 10.1016/j.xphs.2019.03.007.
Kataoka M, et al. Eur J Pharm Biopharm. 2020. 155:29-36. doi: 10.1016/j.ejpb.2020.07.032.
Kataoka M, et al. Mol Pharm. 2021. 18(11):4122-4130. doi: 10.1021/acs.molpharmaceut.1c00537.
Minami K, et al. Pharm Res. 2022. 39(5):977-987. doi: 10.1007/s11095-022-03276-0.
Kataoka M, et al. Mol Pharm. 2023. 20(8):4100-4107. doi: 10.1021/acs.molpharmaceut.3c00237.
Takeyama S, et al. Mol Pharm. 2025. 22(12):7455-7464. doi: 10.1021/acs.molpharmaceut.5c00879.
Masada T, et al. J Pharm Sci. 2026. 115(4):104191. doi: 10.1016/j.xphs.2026.104191
and others
3. Improving oral absorption of poorly absorbed and high-clearance drugs
In general, drugs with high water solubility and relatively large molecular
weights, including peptide drugs, show poor gastrointestinal absorption,
making it difficult to develop oral dosage forms. In recent years, an oral
preparation (Liversus®) of a GLP-1 receptor agonist (semaglutide) using
an absorption enhancer (salcaprozate sodium) has been clinically applied.
However, absorption enhancers cannot apply to all poorly absorbed drugs.
Therefore, it is important to establish a strategy to improve oral absorption
of such drugs without absorption enhancers. Our strategy is to efficiently
deliver such drugs near the intestinal epithelial membrane using liposomes
with various characteristics. Furthermore, we are developing a retentive
sublingual formulation that can avoid the first-pass effect, increase systemic
exposure and improve the absorption of poorly absorbed and high-clearance
drugs.
Publications
Minami K, et al. J Drug Deliv Sci Technol. 2020. 101041. doi: 10.1016/j.jddst.2019.04.035.
Minami K, et al. Yakugaku Zasshi. 2023. 143(4):345-348. Japanese. doi: 10.1248/yakushi.22-00170-1.
4. Isotope-IV study to evaluate Pharmacokinetics
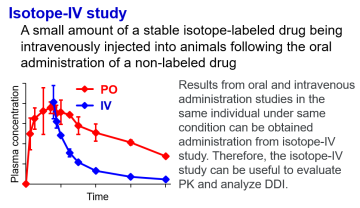 A common method for evaluating Pharmacokinetics (PK) after oral administration
is to evaluate plasma concentration-time profiles after oral and intravenous
administration. However, it is difficult to conduct oral and intravenous
administration studies under the same conditions because pharmacokinetic
changes are often varied especially when drug interactions occur. We believe
that the intravenous administration of stable isotope-labeled drugs following
oral administration of unlabeled drugs (isotope-IV study) can simultaneously
evaluate the systemic clearance and systemic exposure of drugs in the same
body and the same time. Furthermore, this method contributes to reduction
(reducing the number of animals used) in the 3R of animal experiments (replacement,
reduction, refinement). We already demonstrated the usefulness of the isotope-IV
study in PK evaluation for orally administered drugs and in the analysis
of complicated DDIs mediated by CYPs and/or transporters. We are also conducting
research focusing on the metabolites, and are plaining research on the
further usefulness and applicability of this method.
A common method for evaluating Pharmacokinetics (PK) after oral administration
is to evaluate plasma concentration-time profiles after oral and intravenous
administration. However, it is difficult to conduct oral and intravenous
administration studies under the same conditions because pharmacokinetic
changes are often varied especially when drug interactions occur. We believe
that the intravenous administration of stable isotope-labeled drugs following
oral administration of unlabeled drugs (isotope-IV study) can simultaneously
evaluate the systemic clearance and systemic exposure of drugs in the same
body and the same time. Furthermore, this method contributes to reduction
(reducing the number of animals used) in the 3R of animal experiments (replacement,
reduction, refinement). We already demonstrated the usefulness of the isotope-IV
study in PK evaluation for orally administered drugs and in the analysis
of complicated DDIs mediated by CYPs and/or transporters. We are also conducting
research focusing on the metabolites, and are plaining research on the
further usefulness and applicability of this method.
Publications
Kataoka M, et al. Drug Metab Pharmacokinet. 2016. 31(6):405-410. doi: 10.1016/j.dmpk.2016.08.001.
Yamashita S, et al. J Pharm Sci. 2017. 106(9):2671-2677. doi: 10.1016/j.xphs.2017.04.027.
Minami K, et al. J Pharm Sci. 2019. 108(8):2774-2780. doi: 10.1016/j.xphs.2019.03.023.
Minami K, et al. Eur J Pharm Sci. 2020. 152:105409. doi: 10.1016/j.ejps.2020.105409.
Kataoka M, at al. Drug Metab Pharmacokinet. 2023. 51:100514. doi: 10.1016/j.dmpk.2023.100514.
5. Development of a novel Pharmacokinetics booster
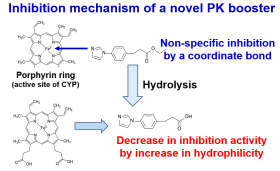
Recently, strategies to improve systemic drug exposure using drug-drug
interactions (inhibition of the drug-metabolizing enzyme cytochrome P450
(CYP)) have been applied clinically (combined use of ritonavir or cobicistat).
However, these often cause serious interactions with other drugs; therefore,
its use is limited (many drugs are contraindicated for use in combination).
We have proposed that a highly safe booster that could solve this problem
would preferably have inhibitory activity that disappears quickly after
inhibiting CYP at a specific site. We have already synthesized and evaluated
compounds that exhibit these characteristics, and have demonstrated the
basic concept. Currently, we are conducting research on the synthesis and
usefulness of compounds aimed at improving inhibitory activity.
Publications
Kawai K, et al. Bioorg Med Chem Lett. 2022. 72:128868. doi: 10.1016/j.bmcl.2022.128868.
Kataoka M, et al. Drug Metab Pharmacokinet. 2024. 56:101005. doi: 10.1016/j.dmpk.2024.101005.
Hoshikawa S, et al. Bioorg. Med. Chem. Lett. 2026. 131:130445. doi: 10.1016/j.bmcl.2025.130445.